Nutritional Requirements
FDA U.S. Food & Drug Administration
 Protecting & Promoting Public Health
Protecting & Promoting Public Health
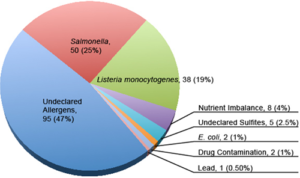
The Reportable Food Registry (RFR or the Registry) was established by Section 1005 of the Food and Drug Administration Amendments Act of 2007 (Pub. L. 110-85), which amended the Food, Drug, and Cosmetic Act (FD&C Act) by creating a new Section 417, Reportable Food Registry [21 U.S.C. 350f]. It required FDA to establish an electronic portal to which reports about instances of reportable food must be submitted to FDA within 24 hours by responsible parties and to which reports may be submitted by public health officials.

FDA Nutritional Label Changes Take Effect in January 1, 2020!
The FDA recently finalized nutrition facts label revisions for all packaged foods. Changes will take effect by January 1, 2020, though manufacturers making less than $10 million in annual gross revenues will have one additional year to comply. These changes reflect diet and disease conscience trends and make room for new scientific information that will help consumers make informed, healthy choices. The FDA website provides an in-depth explanation of the new nutrition label requirements, but for now here are the important points producers need to know to ensure they are on the right path to label compliance.

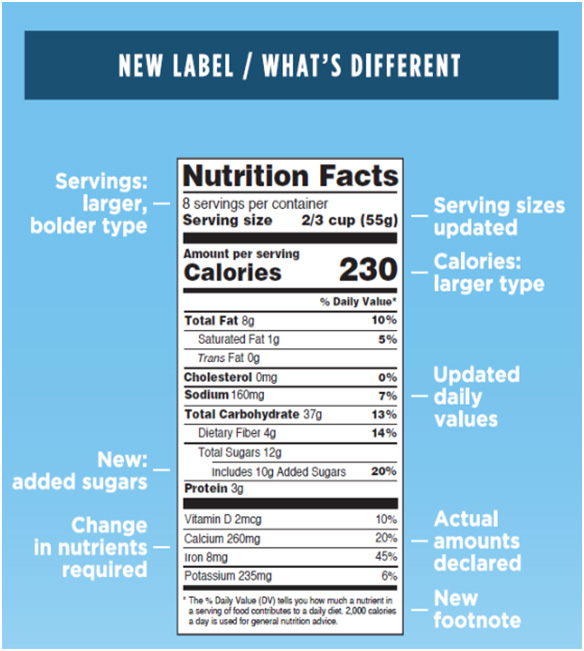
Note: The images above are meant for illustrative purposes to show how the new Nutrition Facts label might look compared to the old label. Both labels represent fictional products. When the original hypothetical label was developed in 2014 (the image on the left-hand side), added sugars was not yet proposed so the “original” label shows 1g of sugar as an example. The image created for the “new” label (shown on the right-hand side) lists 12g total sugar and 10g added sugar to give an example of how added sugars would be broken out with a % Daily Value.
FDA Nutrition Label Revision Explained
- Serving Sizes and Easy Access to Information
- The well-known ‘look’ of the nutrition label will remain the same but important updates will be made to ensure consumers have all the information they need to make mindful decisions about the food they buy. These changes include increasing the type set size for data like calories per serving and serving size.
- Food producers will be responsible for declaring the actual serving amount and percent daily values like calcium, Vitamin D, iron and potassium.
- The label footnote will be revised to better define what ‘Daily Value’ means. It will read: “The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice.”
- Updated Nutritional Science Information
- Daily Values will reflect added sugars. Based on recent scientific data, individuals who count calories to maintain diet are likely ingesting more than 10% of their diet from added, undisclosed sugars.
- Certain nutrients, such as Vitamin A and C, will be required on the label in addition to those already required for compliance (like calcium and iron). Under the previous provision, certain nutrient listings were optional.
- “Calories from Fat” statements were previously required on nutrition labels. Moving forward, calories from fat statements will be removed, while continuing to require “Total Fat“, “Saturated Fat”, and “Trans Fat” on the label; research shows that maintaining these types of fats are the most important to a healthy diet.
- Serving Size & Package Size Requirements
- Certainly the most interesting change to the FDA nutrition label updates is the serving size requirements. Until recently, serving sizes were determined based on amounts of foods and beverages that people ‘should’ be eating. Moving forward, nutrition label updates must reflect the amount of food people are ‘actually’ eating. Serving sizes have changed since they were suggested and published in 1993, with individuals eating larger single serving portions.
- Many packaged foods are generally consumed in a single serving, though intended for several servings. The FDA requires packaged foods to list nutritional information that reflects the nutritional information for the total consumable package, apart the recommended serving size. An example of multi serving products generally consumed in a single serving would be ‘Big Grab’ potato chips and packaged nuts. This new label format is termed “dual column” and aims to help consumers better understand portion sizes.
These updated FDA Nutrition Label requirements means big changes for the majority of packaged food manufacturers. These changes require updates to nutrition information and label design. In some cases, manufacturers will be required to evaluate and update several label versions to accommodate their seasonal product lines and flavor options. For more information about meeting the new FDA nutrition label requirements, graphic revisions and what print technology best suits your application, please contact Met Speed Label at www.metspeedlabel.com or call (215) 946-7200
Download a PDF version of this page or Contact Us for more information!
